acetic acid and sodium hydroxide balanced equation|acetic acid naoh : Pilipinas CH 3 COOH + NaOH → NaCH 3 COO + H2O Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by . Tingnan ang higit pa Manage your employees with JYOSNA , a powerful and secure enterprise solution.
PH0 · acetic acid naoh
PH1 · acetic acid and naoh equation
PH2 · Iba pa
Grab the hottest Abella Anderson nude pictures right now at PornPics.com. New FREE naked Abella Anderson porn photos added every day.
acetic acid and sodium hydroxide balanced equation*******Balanced Chemical Equation. CH 3 COOH + NaOH → NaCH 3 COO + H 2 O. Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by-step answer, or balance another equation. Reaction Information. Word Equation. Acetic Acid + Sodium Hydroxide = Sodium Acetate + Water. Tingnan ang higit pa
CH 3 COOH + NaOH → NaCH 3 COO + H2O Equation is already balanced. ⬇ Scroll down to see reaction info and a step-by . Tingnan ang higit paTo balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. The balanced equation will appear above. Use uppercase for the first character in the element and lowercase for the second character. . Tingnan ang higit paWord Equation CH3COOH + NaOH = NaCH3COO + H2O is a Double Displacement (Metathesis) reaction where one mole . Tingnan ang higit paRead our article on how to balance chemical equations or ask for help in our chat. Balance any equation or reaction using this chemical equation balancer! Find out what type of reaction occured. You can also ask for help or report an issue with . Tingnan ang higit pa Acetic acid, "CH"_3"COOH", will react with sodium hydroxide, "NaOH", to produce sodium acetate, "CH"_3"COONa", and water. The unbalanced chemical .Balanced equation for acetic acid CH 3 COOH with Sodium hydroxide NaOH. When Acetic acid CH 3 COOH reacts with Sodium hydroxide NaOH, the formation of S odium acetate .The reaction of Sodium hydroxide and Acetic acid (also called Ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. Strong bases are . There are three main steps for writing the net ionic equation for Acetic acid and Sodium hydroxide. First, we balance the molecular equation. Second, we writ. Write the balanced equation for the neutralization reaction between aqueous sodium hydroxide and acetic acid. The Molarity of Acetic Acid in Vinegar. Use your two best sets of results (with the . When acetic acid reacts with sodium hydroxide, an aqueous solution of sodium acetate is formed along with water. Since this reaction is an acid-base neutralization reaction, and these reactions go to completion, instead of using an equilibrium arrow, .
Explanation: acetic acid (ethanoic acid): CH 3COOH. CH 3COOH = C2H 4O2. sodium hydroxide: N aOH. neutralisation reactions always produce a salt and .
A molecular equation is sometimes simply called a balanced equation. In a molecular equation, any ionic compounds or acids are represented as neutral compounds using .Write a balanced molecular equation, a total ionic equation, and a net ionic equation for the reaction between HNO 3 acid and Ca(OH) 2 Answer Molecular equation: 2HNO 3 (aq) .
Write the balanced equation for the neutralization of acetic acid by sodium hydroxide. Be sure to use subscripts correctly and include all phase labels. Write the balanced chemical equation for the reaction of sodium bicarbonate with aqueous acetic acid to produce carbon dioxide, water, and sodium acetate. 1. For the reaction of acetic acid, .
To write the net ionic equation for the reaction between acetic acid and sodium hydroxide in a mathematical form, you need to first write the balanced chemical equation. The balanced equation for this reaction is CH3COOH (acetic acid) + NaOH (sodium hydroxide) -> CH3COONa (sodium acetate) + H2O (water). Next, you need to identify .
C_2H_4O_2 + NaOH = C_2H_3O_2Na + H_2O acetic acid (ethanoic acid): CH_3COOH CH_3COOH = C_2H_4O_2 sodium hydroxide: NaOH neutralisation reactions always produce a salt and water. in this case, the salt is sodium ethanoate (CH_3COONa, C_2H_3O_2Na) equation: C_2H_4O_2 + NaOH = C_2H_3O_2Na + . Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 14.6.1 14.6. 1 ). A solution of acetic acid ( CH3COOH CH 3 COOH and sodium acetate CH3COONa CH 3 COONa) is an example of a buffer that consists of a weak acid and its salt.

In other words: Cl – (aq) + H+ (aq) → HCl (aq) Therefore the acid and base that result in the formation of sodium chloride by neutralization reaction are: HCl (aq) + NaOH (aq) → H 2 O + NaCl (aq) Exercise 7.17.1 7.17. 1. For each of the following, write a balanced neutralization equation: The reaction of calcium hydroxide with .
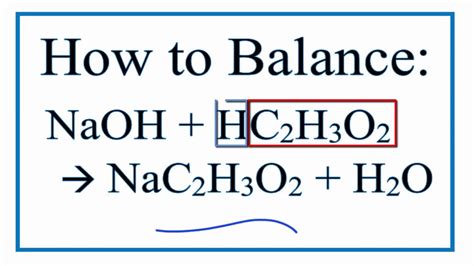
Here is the balanced chemical equation for the reaction and a closer look at the steps involved. Balanced Chemical Equation for Baking Soda and Vinegar Reaction. One mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one .acetic acid naoh The reaction of Sodium hydroxide and Acetic acid (also called Ethanoic acid) represents a net ionic equation involving a strong base and a weak acid. This reaction is considered a neutralization reaction. The base (NaOH) and weak acid (CH3COOH) react to produce a salt (NaNO3 and water (H2O). What happens when .Write the complete balanced equation for the neutralization reaction of acetic acid and sodium hydroxide. 2. Calculate the equivalence point for this titration. Analyte solution: 25 mL of 0.1M acetic acid. Titrant: 0.1 M sodium hydroxide. There are . When equal amounts of a strong acid such as hydrochloric acid are mixed with a strong base such as sodium hydroxide, the result is a neutral solution. The products of the reaction do not have the characteristics of either an acid or a base. The balanced molecular equation is:acetic acid and sodium hydroxide balanced equation acetic acid naoh A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or a buffer. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 7.1.1 7.1. 1 ). A solution of acetic acid ( CH3COOH CH 3 COOH and sodium acetate . What are the molecular, ionic, and net ionic equations for the reaction of acetic acid #"HC"_2"H"_3"O"_2# with potassium hydroxide? Chemistry Chemical Reactions Chemical Equations 1 AnswerWord Equation. Acetic Acid + Sodium Hydroxide = Water + Sodium Acetate. HC2H3O2 + NaOH = H2O + NaC2H3O2 is a Double Displacement (Metathesis) . Since there is an equal number of each element in the reactants and products of HC2H3O2 + NaOH = H2O + NaC2H3O2, the equation is balanced. Balance HC2H3O2 + NaOH = H2O + .acetic acid and sodium hydroxide balanced equationSteps in Balancing a Chemical Equation. Identify the most complex substance. Beginning with that substance, choose an element (s) that appears in only one reactant and one product, if possible. Adjust the coefficients to obtain the same number of atoms of this element (s) on both sides.
Word Equation. Sodium Hydroxide + Sulfuric Acid = Sodium Sulfate + Water. NaOH + H2SO4 = Na2SO4 + H2O is a Double Displacement (Metathesis) reaction where two moles of aqueous Sodium Hydroxide [NaOH] and one mole of aqueous Sulfuric Acid [H 2 SO 4] react to form one mole of aqueous Sodium Sulfate [Na 2 SO 4] and two moles of liquid .When sodium hydroxide solution is treated with acetic acid to form sodium acetate and water. The following reaction takes place: $$ NaOH + CH_3COOH \rightarrow CH_3COONa + H_2O $$ The above reaction is a type of neutralization reaction/ double displacement reaction. When acetic acid reacts with sodium hydroxide, an aqueous solution of sodium acetate is formed along with water. Since this reaction is an acid-base neutralization reaction, and .Write the balanced chemical equations for the following reactions and identify the type of reaction in each case. (i) nitrogen gas is treated with hydrogen gas in the presence of a catalyst at 773 K to form ammonia gas. (ii) Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water.
My Talking Angela 2+ is the virtual pet game that makes every day more stylish and fun. Players help this fashionable cat stay busy in her big-city home. - Awesome hair, makeup and fashion choices. - Epic activities, like dancing, baking, and martial arts. - Delicious food and snacks. - Jet-setting travel options.
acetic acid and sodium hydroxide balanced equation|acetic acid naoh